iQ-4VIEW VET Zero Footprint Viewer: IQ-WEB Plugin
Description
IMAGE Information Systems iQ-4VIEW VET Zero Footprint Viewer for Concurrent Web Users
Note: This is an iQ-WEB Plugin and requires iQ-WEB as well
DIAGNOSTIC* ZERO-FOOTPRINT READING AT HIGH SPEED
. Multi-modality thin-client viewer for nearly any browser and operating system
. Runs on virtually any device (e.g. computer, tablet, smartphone)
. No installation on the client required
IQ-4VIEW VET Features
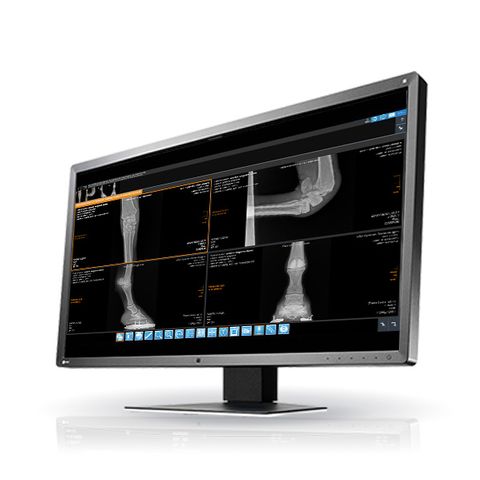
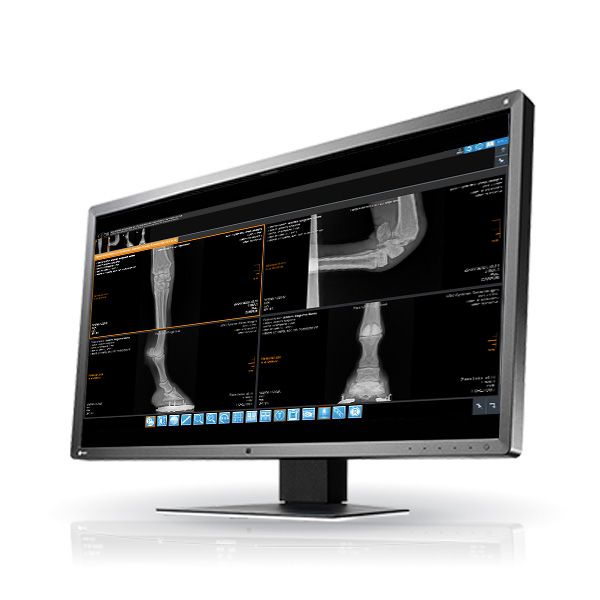
iQ-4VIEW WEB VIEWER
- View any kind of images, Structured Reports, and Encapsulated PDFs
- Typical time to first image < 3 seconds
- Thumbnail previews for easy selection of series via drag & drop
- Displays scoutlines of cross-sectional images
- Center/window, color remappings
- Stack mode/cine mode (up to 50 fps)
- Measurements (distance, ROI, angle, ratio, Cobb angle, hip dysplasia angle, VHS (Vertebral Heart Score / Vertebral Heart Size index), TPLO, TTA, Georgees Line, cervical curve, lumbar curve, Gonstead analysis, Spine Labelling (NEW!)
- Annotations (oval/circular, polygonal, line and arrow) (NEW!)
- Compare multiple studies
- Creation of DICOM Secondary Capture images (from active views, JPEG files or tablet / smartphone cameras) (NEW!)
- Easy synchronization of series, e.g. for comparing CT scans
- Web-based creation of DICOM Structured Reports with UTF-8 support
- Zero-footprint tomosynthesis
- Two image processing modes (client/server) for fast and slow network
- Background caching
- PNG (lossless) / JPEG (lossy) image data compression
- License model: 1 license per iQ-WEBX server with a defined number of concurrent users (5, 10, 15, 20 or unlimited)
- Languages: English, French, German, Russian, Spanish
- Annual Service Package: mandatory (see Warranty and Service Packages for details)
- Includes 1 years software refresh.
LICENSING
- Available for 2, 5, 10, 15, 20 or an unlimited number of concurrent users iQ-WEB2GO additionally suggested if accessing iQ-4VIEW from small-sized portable devices
- Report editor is included
CERTIFICATION
- CE 0482 and FDA 510 (k)